Abstract
Introduction: Current management of patients diagnosed with paroxysmal nocturnal hemoglobinuria (PNH) includes C5 complement inhibitors such as ravulizumab. The ravulizumab dosage regimen varies by patient body weight and consists of a single loading dose followed by maintenance doses administered every 8 weeks via intravenous infusion (see Table for label-recommended loading and maintenance dosing regimens by patient body weight category). Given its recent 2018 FDA approval, real-world data on the dosing patterns of ravulizumab are limited. This study investigated the real-world dosing patterns of patients with PNH treated with ravulizumab in a large US population.
Methods: This retrospective longitudinal cohort study was conducted using provider-based claims data from the Symphony Health Integrated Dataverse ® (IDV). Patients were ≥12 years of age and received ≥2 infusions of ravulizumab between June 21, 2019 and May 6, 2021. The index date was defined as the 1st medical claim for ravulizumab infusion with ≥6 months of continuous clinical activity prior to the index (the baseline period). Patients with ≥1 diagnosis of atypical hemolytic uremic syndrome (another indication for ravulizumab) during the baseline period or on the index date were excluded to identify those with PNH. Patient baseline demographic and clinical characteristics including PNH-related comorbidities and symptoms were described. Because Symphony IDV does not record patient weight, it was assumed that all patients had a weight ≥60 and <100 kg (ie, "medium body weight"), consistent with mean weights reported in recent clinical trials of ravulizumab (~70 kg) and in the US population (men: 91 kg; women: 77 kg). Mean and mode loading and maintenance doses as well as the proportion of patients with high, label-recommended, and low loading and mean maintenance doses were calculated. To test the weight assumptions, 2 sets of sensitivity analyses were carried out to account for alternative dosing pattern scenarios, with all patients classified as "low body weight" (≥40 and <60 kg) or "high body weight" (≥100 kg).
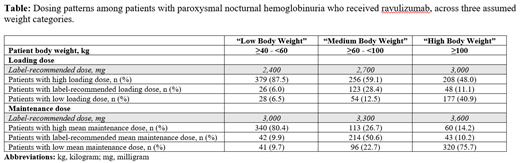
Results: A total of 433 patients with PNH were included in the study; the mean age was 47 years and 52% were female. The mean treatment period was 11.8 months; and 54%, 40%, and 6% of patients initiated ravulizumab in 2019, 2020, and 2021, respectively. Most patients (76%) were insured through a commercial insurance plan. Aplastic anemia and myelodysplastic syndrome were present at baseline in 22% and 5% of patients, respectively. The most frequent comorbidities were coagulopathy (13%), deficiency anemias (12%), and hypertension (11%); and 40% of patients had anemia (other than aplastic anemia) at baseline. Prior to initiating ravulizumab, 42% of patients had previously received ≥1 infusion of eculizumab during the baseline period. The mean (standard deviation [SD]) and mode loading doses were 3,316 (2,932) and 3,300 mg, respectively. The proportion of patients with a high, label-recommended, and low loading dose was 59%, 28%, and 13%, respectively (Table). During the maintenance phase, the mean (SD) and mode doses were 3,404 (1,024) and 3,300 mg, respectively; the proportion of patients with a high, label-recommended, and low mean dose during this phase was 27%, 51%, and 23%, respectively (Table). The sensitivity analyses revealed similar trends: nearly half or more than half of patients received a high loading dose; the proportion with a high mean dose during the maintenance phase was substantial when it was assumed that all patients had "low body weight" and negligible when assuming "high body weight" (Table).
Conclusions: Most patients with PNH treated with ravulizumab received a higher than label-recommended loading dose regardless of weight category. Additionally, the mean dose received during the maintenance phase was higher than the label-recommended dose of 3,300 mg in more than one-quarter of patients (≥60 - <100 kg). The deviations from label-recommended dosing regimens-especially dosages exceeding recommendations-suggest that in some patients, PNH is not controlled by ravulizumab. Alternative weight scenarios will be tested in future analyses to account for weight distribution in the US. Future budget impact analyses of ravulizumab in patients with PNH should consider real-world dosing patterns in order to determine cost vs benefit.
Cheng: Novartis: Other: I am an employee of Analysis Group, a consulting company that received funding from Novartis for this research study.. Fishman: Apellis: Current Employment, Current equity holder in publicly-traded company. Sarda: Apellis: Current Employment, Current equity holder in publicly-traded company. Krishnan: Apellis: Current Employment, Current equity holder in publicly-traded company. Kunzweiler: Apellis: Other: Employee of Analysis Group, Inc., Research Funding. Vu: Apellis: Other: Employee of Analysis Group, Inc., Research Funding. Yenikomshian: Apellis: Other: Employee of Analysis Group, Inc., Research Funding. Duh: Novartis: Other: I am an employee of Analysis Group, a consulting company that received funding from Novartis for this research study..

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal